Short answer, yes! Tedizolid (SIVEXTRO™) is a novel oxazolidinone antibiotic approved recently for acute bacterial skin and skin structure infections (ABSSSI) and I believe it is a matter of time until being approved for other indications like pneumonia (HAP/VAP).
But is it really with additional benefits over Linezolid ?
Short answer, No!
So I am going to assess it through the claimed benefits of Tedizolid over Linezolid!
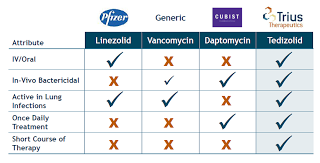
Difference between Linezolid and Tedizolid :
Antimicrobial Activity :
Bactericidal VS bacteriostatic is still a controversy but there is no doubt in certain cases a bactericidal agent is recommended over bacteriostatic agent like in case of :
- Meningitis.
- endocarditis.
- Febrile neutropenia.
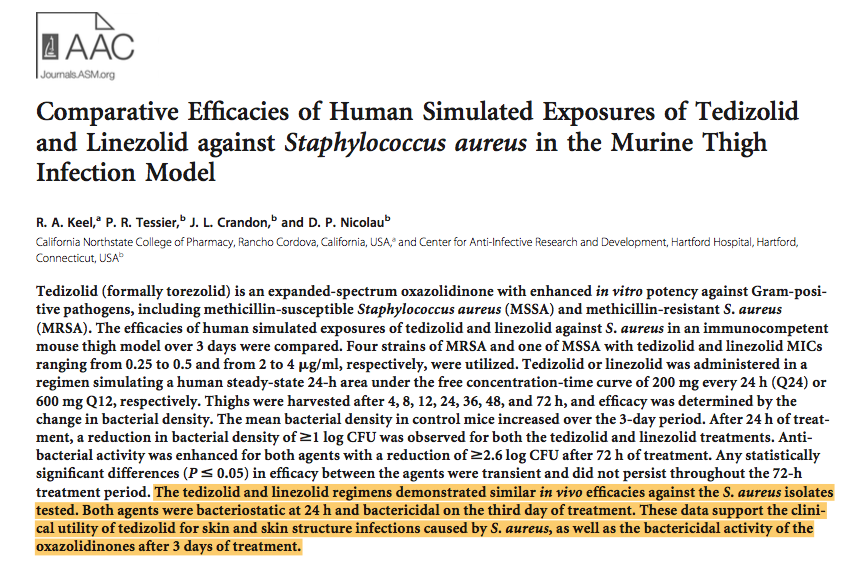
So according to the below paper published in AAC journal, The group oxazolidinone has the same anti-bacterial properties.
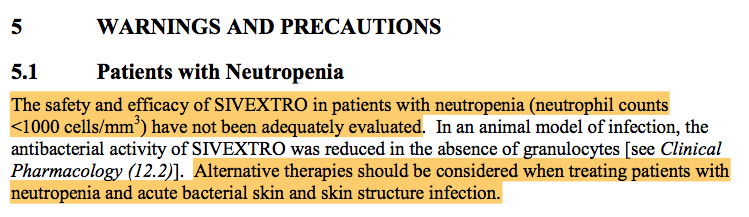
But let us consider that Tedizolid has an invivo bactericidal activity, what about neutropenic patients in which such agents are badly required!
OK, the answer from Tedizolid insert package which IS NOT in Linezolid precautions and warnings section :
Once daily dosing :
Yes Tedizolid is a once daily 200mg for both IV and oral form but does it matter for an acute bacterial skin and skin structure infections ?
I believe this kind of feature is to certain extend important for chronic diseases like diabetes or hypertension because the patient will go under these kind of drugs for a considered period of his life not just a 6 or 7 or even 14 days of infection treatment that might required hospitalization.
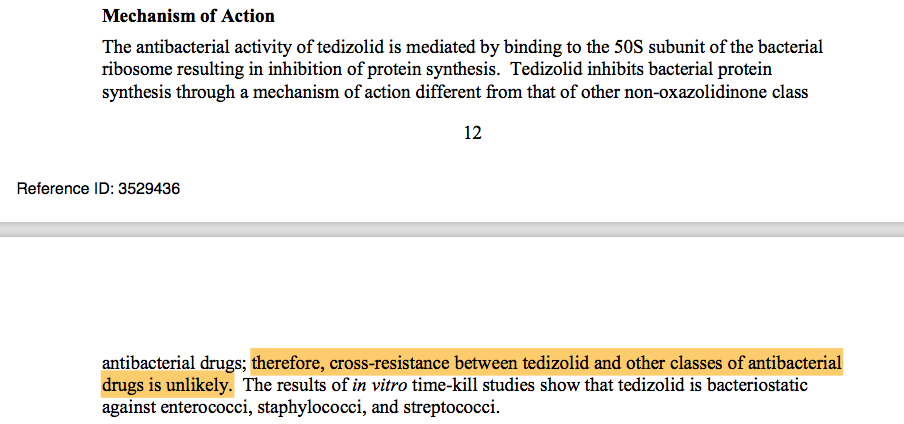
Tedizolid is a novel agent and has no emerging resistance :
I totally agree with that but do you know in the insert leaflet of Tedizoild there is a nice sentence :
So generally if Linezolid is abused and there is a high resistance prevalence in a clinical setting or in certian patients, there is a good chance that Tedizolid will have a resistance soon.
It has no SSRI drug-drug interactions :
The marketing company claims that there is no any warnings or precautions with SSRI. Yes that is right but WHY? as I see many physicians even with infectious diseases departments did not know why!
Well, the answer from the package insert directly to you:
So you don’t have any kind of data related to such potential drug drug interaction! so that doesn’t mean by default that Tedizolid is safer than Linezolid! it is just no enough data about it.
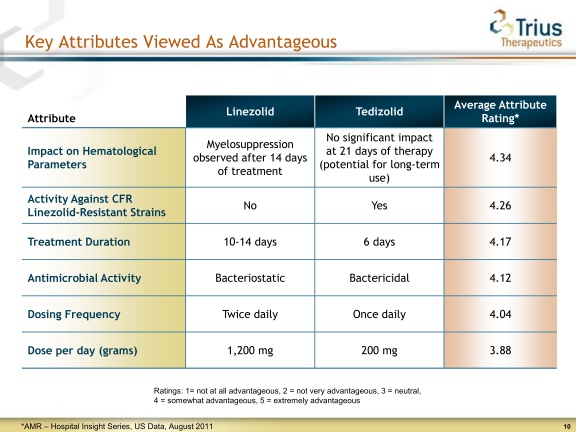
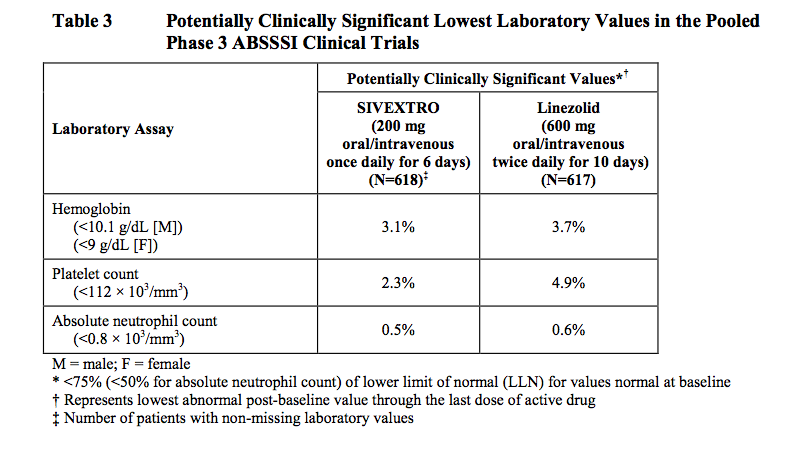
The Hematological Parameters :
Hematology laboratory abnormalities that were determined to be potentially clinically significant in the pooled Phase 3 ABSSSI clinical trials are provided as follow :
 So, the percentage at the end of treatment (Non Inferiority results) ? so what about Linezolid data at the 6th day or what about the clinical success rate at the 6th day ?
So, the percentage at the end of treatment (Non Inferiority results) ? so what about Linezolid data at the 6th day or what about the clinical success rate at the 6th day ?
No data!
But anyway as stated for the Myelosupression in Tedizolid insert :
In the Phase 3 trials, clinically significant changes in these parameters were generally similar for both treatment arms (see Table 3).
Peripheral and Optic nerve neuropathy
In Phase 3 trials, reported adverse reactions for peripheral neuropathy and optic nerve disorders were similar between both treatment arms (peripheral neuropathy 1.2% vs. 0.6% for tedizolid phosphate and linezolid, respectively; optic nerve disorders 0.3% vs. 0.2%, respectively). No data are available for patients exposed to SIVEXTRO for longer than 6 days.
Although Tedizolid is numerically higher but both arms are the same in such adverse reactions.
Conclusion
I think Tedizolid is just a more potent Linezolid and this is might be related to the chemical structure (SAR). It does the job in a shorter period of time ( 6 days ) with the same all other clinical parameters. So there is no data up to date for using Tedizolid more than 6 days! it might be an advantage but also it should be considered as a limitation because what if it doesn’t treat the infection within the 6 days? yet it is approved in one indication (ABSSSI) and I believe it is a matter of time till to be approved in other indications, for example HAP/VAP and if the marketing company will just leave it for one indication they will fail! because Ceftaroline is coming for ABSSSI with MRSA activity!
Again if they got approval for more indication like HAP/VAP, the recommended treatment duration by ATS/IDSA is 7 days! so I believe Tedizolid will have a hard time soon unless they do more trails for longer duration of treatment but then they will lose their claimed marketing advantage of being 6 days only with once daily dose.